26Al
This enters the oceans in ionic form and mixes with stable 27Al, later becoming adsorbed on various particles. Its concentration rises with depth and its half-life is 0.716•106 years. Its residence time was estimated as 1400 years and that makes it valuable for dating authigenic minerals, although detecting it is not easy. It has been employed to determine the rates of growth of manganese nodules of which one estimate is 2.3 mm My-1. When it is measured together with 10Be from the same sample, the ratio of their respective rates of radioactive decay decreases with time. This geochronometer is useful because the time-dependent variations in the rates of production and the geochemical paths of the two nuclides tend to annul each other (Lal and Somanyajulu 1984).
39Ar
This radionuclide forms in the atmosphere through the nuclear reaction
40Ar(n,2n)39Ar
and decays by beta emission to 39K with a half-life of 269 years.
Its contribution to atmospheric radioactivity is about 1.87 ± 0.17 Bq m-3 and of this, less than 5% is due to thermonuclear testing. It has a long residence time that damps down fluctuations in its production rate and contributes to probable constancy of its concentration in terms of time and latitude. This makes it valuable for dating for instance glacial ice. Air bubbles containing 39Ar may become trapped in the ice and can be analyzed to assess its disintegration rates as well as that for 14C and 81Kr.
39Ar may also be used for dating of groundwater or groundwater recharge. Because argon, like other inert gases, occurs mostly in the atmosphere, concentrations in groundwater depend on the time elapsed since the water was in contact with the atmosphere.
There are several problems connected to the use of this method. One is that argon is an inert gas and is not easily chemically trapped. Therefore, it may be partly removed from the system under study by molecular diffusion.
Another difficulty is that analyzing 39Ar entails collecting very large volumes of water and requires a quantitative extraction of argon from this water volume.
Finally, excess 39Ar may arise from 39K as shown in Eq. (1), i.e. through the (n,p) reaction
39K( n,p )39Ar
in rocks containing measurable amounts of uranium and thorium who are responsible for the neutron flux.
7Be
7Be occurs naturally and is radioactive with a half-life of 53.29 days. It decays to stable 7Li by electron capture. 7Be is produced in the atmosphere by high-energy spallation reactions between cosmic-ray neutrons and nitrogen in the atmosphere about 1.5 km above the surface of the Earth. It forms BeO molecules which diffuse until encountering dust particles to which they adhere. Dust particles are again important as nucleation points for ice and/or water. This leads in turn to precipitation of snow or rain, now incorporating 7Be. Once deposited on the terrestrial surface, it enters the geochemical cycle of beryllium. Although it has a low production rate, it is easy to detect because it is found practically carrier-free in the atmosphere. Years ago, it was suggested that 7Be might be useful in dating and its rapid radioactive decay facilitates examining various atmospheric processes, surface water in the oceans and the mixing of sediments in off-shore marine and lake environments.
10Be
Like 7Be, 10Be is a spallation product formed by cosmic rays in the atmosphere and it descends in precipitation to reach sea bottom sediments and continental ice sheets. Its half-life is 1.5•106 years and its global precipitation rate has been estimated as between 1.5•10-2 and 3•10-2 atoms cm2 s-1, although a lower value has been claimed for Antarctic snow. It is introduced into waters as BeO or in ionic form and mixes with the stable isotopes 9Be and 27Al. Having high ionization potentials, they may become adsorbed on both organic and inorganic particles. Seawater has a very low concentration of 10Be compared to river waters and lower concentrations at the surface than in deep waters. The residence time in the waters has been calculated as being about 1600 years. Therefore, it is useful for dating authigenic minerals which take it up in ionic solution in the oceans. But there are difficulties because of its low level of activity. In addition, its disintegration rate varies sporadically with depth in cores (associated abundances depending not just on the time elapsed since deposition, but on the rate of deposition and secular variations in the production rates). It cannot be assumed that the rates of sedimentation in oceans at any particular place were constant for geologically significant time intervals of ~ 106 y. As well as sediment dating in oceans, 10Be concentrations on land, for instance in soils, can be examined. However, few data are available again due to the low activities involved.
36Cl
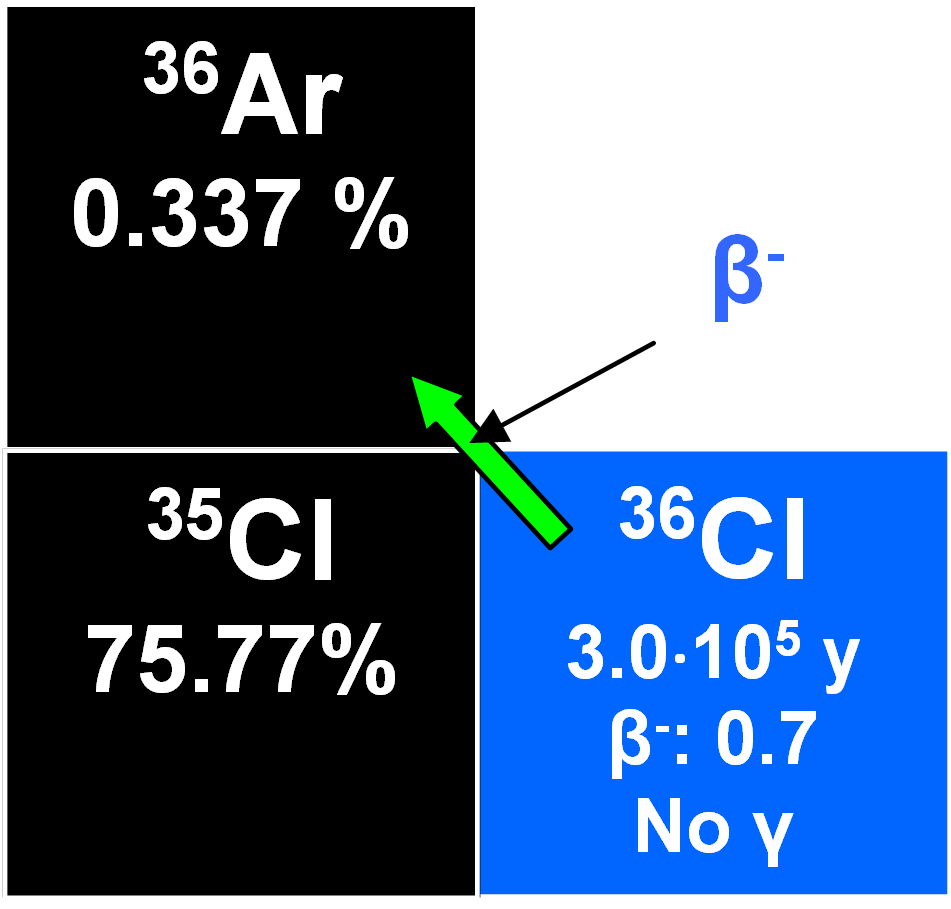
36Cl is produced in the atmosphere through the nuclear reaction:
36Ar(n,p)36Cl,
Mostly in the stratosphere and its formation is latitude-dependent because of the effects of terrestrial magnetism on cosmic ray intensities. Mixing is rapid and the concentration of 36Cl is virtually constant. It attaches to atmospheric aerosols in the sub-micron range and these are progressively removed by precipitation from the lower troposphere. Entry from the stratosphere into the troposphere varies seasonally and the fallout rate is greater in middle latitudes than it is at the poles or equatorial. Its precipitation rate was determined at 57° N latitude at Stripa, Sweden, as about 14 ± 3 atoms m -2s -1 (Lal and Peters 1967). Its half-life is 3.01•105 y. This makes it useful for dating glacial, groundwater, etc. Neutron irradiation of oceanic aerosols containing NaCl following thermonuclear testing at low altitudes induced increased fallout through the nuclear reaction 35Cl(n,y)36Cl, an effect which has increased from 1953 to a 1964 maximum of 70 000 atoms m-2s-1 (Bentley et al. 1986). Because such tests began earlier over oceans, the 36Cl bomb-induced peak predated that of tritium by about ten years. A residence time in the atmosphere for chlorine ions of less than three years has been proposed which may be why the bomb pulse of chlorine-36 was transient with fallout rates returning to normal by the early 1970's when low altitude testing was terminated (Elmore et al. 1982). This bomb-induced pulse was used to study the migration of water through the unsaturated zone into groundwater aquifers and also the stratification of waters in unconfined aquifers.
There is also a subterranean production of 36Cl, and the world average chlorine contents of granite and basalt have been given as ~ 50 ppm and ~ 200 ppm, respectively. Sedimentary rocks have variable contents ranging from 10 ppm in sandstones to 20 000 ppm in deep-sea limestone. Rock outcrops are exposed to the cosmic neutron flux so that some 36Cl results from neutron capture by 35Cl, but, below a few meters, it is ineffectual. Nonetheless, some 36Cl results from an in situ neutron flux in rock matrices caused by spontaneous fission neutrons from 238U, from neutron-induced fission of 235U and from (α,n)-reactions triggered by alpha-particles from uranium and thorium natural radioactive decay series. This flux may be of the order of 10-4 cm-2s-1 (Kuhn et al. 1984).
81Kr and 85Kr
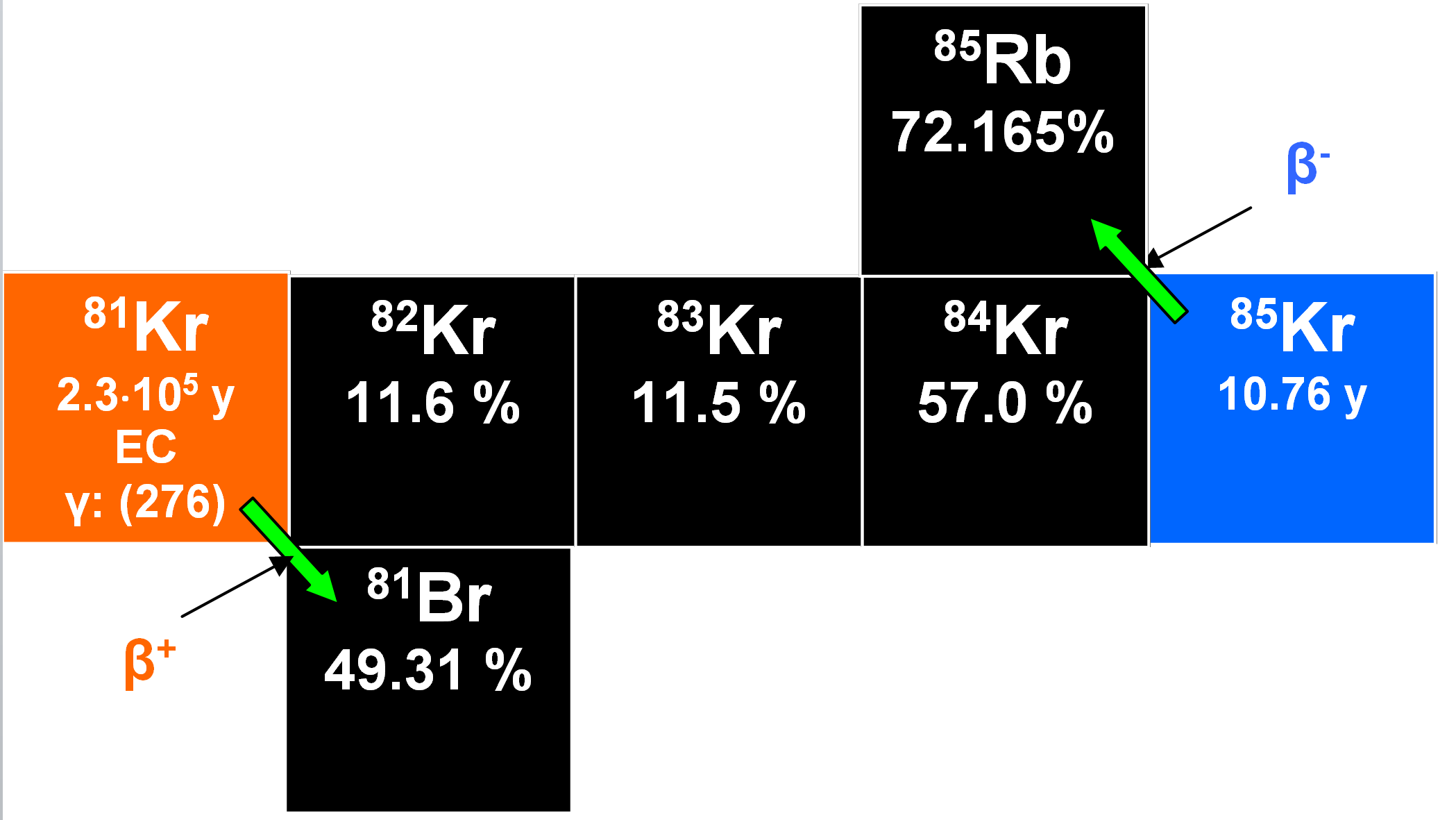
81Kr resembles both 39Ar and the short-lived 37Ar in that it is a cosmogenic radionuclide occurring in the atmosphere, in groundwater that has been in contact with the atmosphere and in air occluded in glacial ice. Both 81Kr and 85Kr result from spallation reactions involving protons and also from (n,y) reactions from the stable 80Kr and unstable 84Kr. 81Kr has a half-life of 2.13•105 y and undergoes radioactive decay by electron capture to the stable 81Br. It is not a fission product, and has an activity of 0.067 ± 0.003 dpm /L. This value exceeds expectations by a factor of up to two that might imply an extra-terrestrial influence of some sort (Kuzimov and Pomansky 1980). 81Kr has been used to date glacial ice.
85Kr is a fission product continuously being released into the atmosphere during the reprocessing of spent nuclear fuel. Because major reprocessing plants are located in the northern hemisphere, 85Kr concentrations in the southern hemisphere are systematically lower. In addition, part of the 85Kr released at midnorthern latitudes decays before it can be transported to the southern hemisphere. It has a half-life of 10.76 y and decays to the stable nuclide 85Rb by beta emission. The specific activity of anthropogenic 85Kr has been reported as about 3•104 dpm liter-1 (Alburger et al. 1986).
32Si
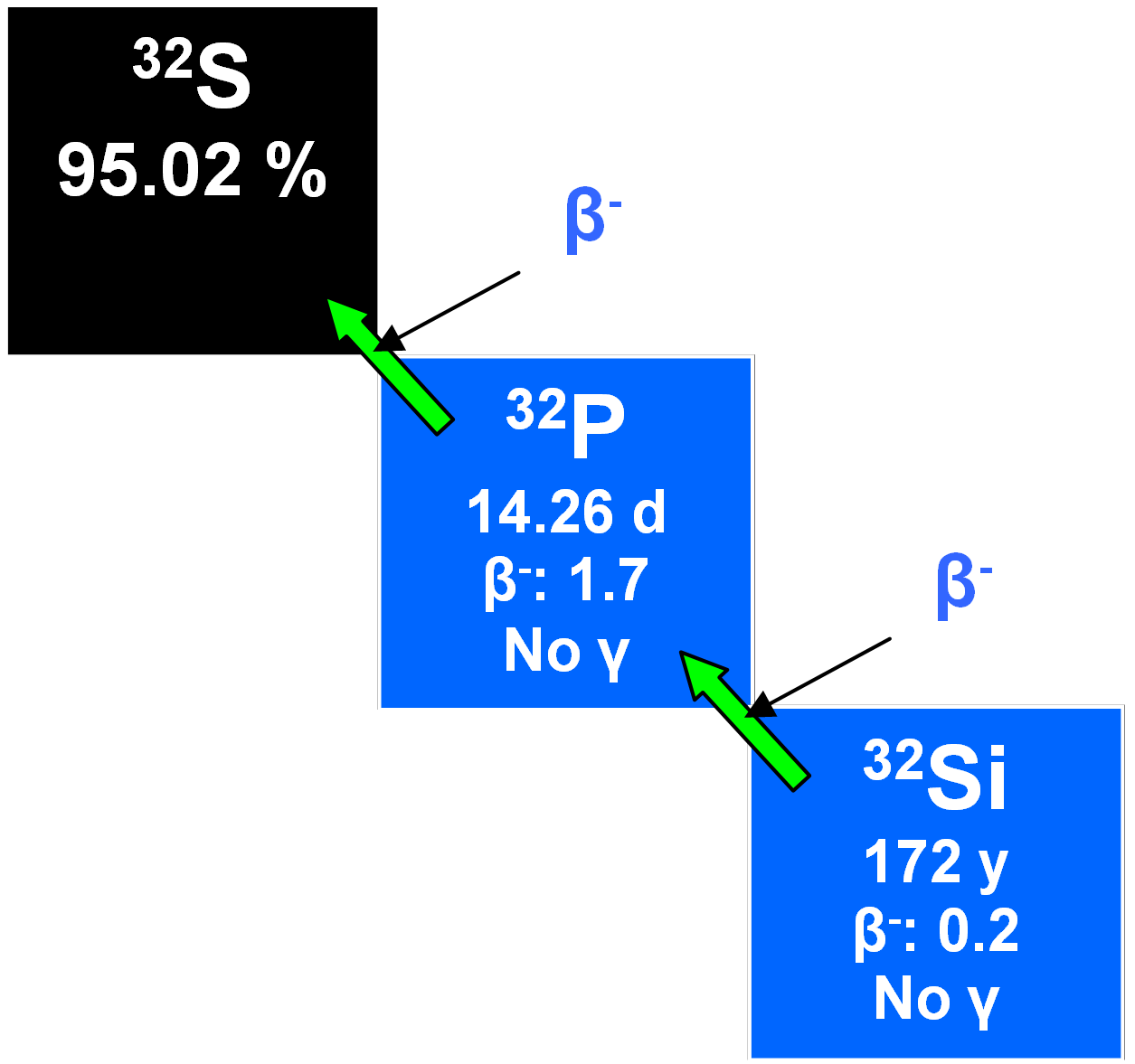
32Si is produced by the cosmic ray spallation of argon in the upper atmosphere from whence it descends to mix with surface water so that, as a radiotracer, it may be applied to measuring ocean water mixing and currents, glacier formation, the accumulation of sediments and the stability of groundwater over centuries. However, there is a problem with the half-life of which estimates range from 60 to 700 years or more. A fairly recent value was 172 ± 4 y (Alburger et al. 1986). Of course, this limits its utility and another disadvantage is that its extremely low concentration in planetary waters requires using large samples of around ten tonnes of water for analytical purposes.